Application and Technology News

Mitigating Hazardous Chemicals with Isolation Technology
The entire process of pharmaceutical manufacturing involves a whole list of meticulous guidelines, both local and international, to adhere to. Moreover, it needs to pass the different stages of production before it can reach market platforms, and only then can the industry be assured of their product’s quality.
Lessen Bioburden: Control in the Biopharmaceutical Business
There have been many great feats made by the pharmaceutical industry in the past few years, but there is one that bothers both the healthcare and manufacturing industries continuous soar, the possibility of health decline and a high risk of bioburden.
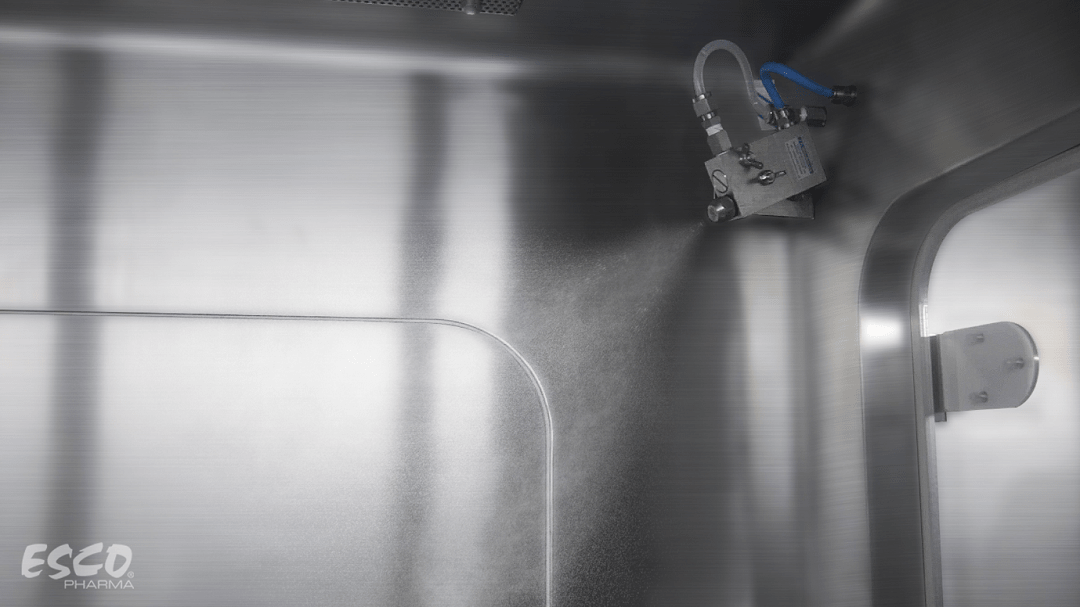
Biodecontamination Efficiency: Get to Know the BioVap™
Contamination control is of extreme importance in biopharmaceutical industries, especially in maintaining product quality and sterility. As standards and guidelines evolved over time, biodecontamination became a part of the standard operating procedures (SOPs) for facilities who manufacture and handle high-quality products.

Quality Makes Pharmaceutical Industries
Pharmaceutical industries need to gain a high degree of assurance that their manufacturing processes are consistently producing marketable drug products with the necessary attributes of identity, strength, quality, purity, and potency.
The Breakdown of Drug Discovery & Development
Novel drugs do not just appear overnight, rather, they are borne from the ceaseless sacrifice of dedicated researchers. The story starts with exhaustively studying a promising compound and ends with a safe and properly documented final drug product in the market.

Prioritizing Personnel Safety in Antibiotic Production
Recent studies on antibiotic occupational exposure has shown evidences of high AMR levels and various adverse health effects among production line workers without proper equipment to isolate them from the process.

The GMP Know-Hows
GMP, or Good Manufacturing Practice is one of the most important guidelines followed in the field of pharmacy. It tackles all aspects of production - from the raw materials, the facility, to the equipment.

Kilo Labs: From small to Big!
Kilo labs, also called pilot scale manufacturing, are able to mimic plant conditions to ensure that the desired form or polymorph of the active pharmaceutical ingredient is obtained.





