Application and Technology News

R&D Laboratory Containment Isolator Made Its Way to Big Pharma
Esco Pharma USA factory successfully completed the design, ergonomic trials, manufacture, assembly, testing, and delivery of a 14-glove double-sided R&D Laboratory Containment Isolator for one of the largest pharmaceutical manufacturing companies in the world.

The Need for Containment on High Potency Active Pharmaceutical Ingredients
High Potency Active Pharmaceutical Ingredients (HPAPIs) are drugs that represent a pivotal change in the new generation of developing patient therapies.

Radiopharmaceutical Products: The Origin
According to the World Health Organization (WHO), the scope of radiopharmaceutical products include organic and inorganic compounds, peptides, proteins, monoclonal antibodies and fragments, and oligonucleotides labelled with radionuclides with half-lives from a few seconds to several days.
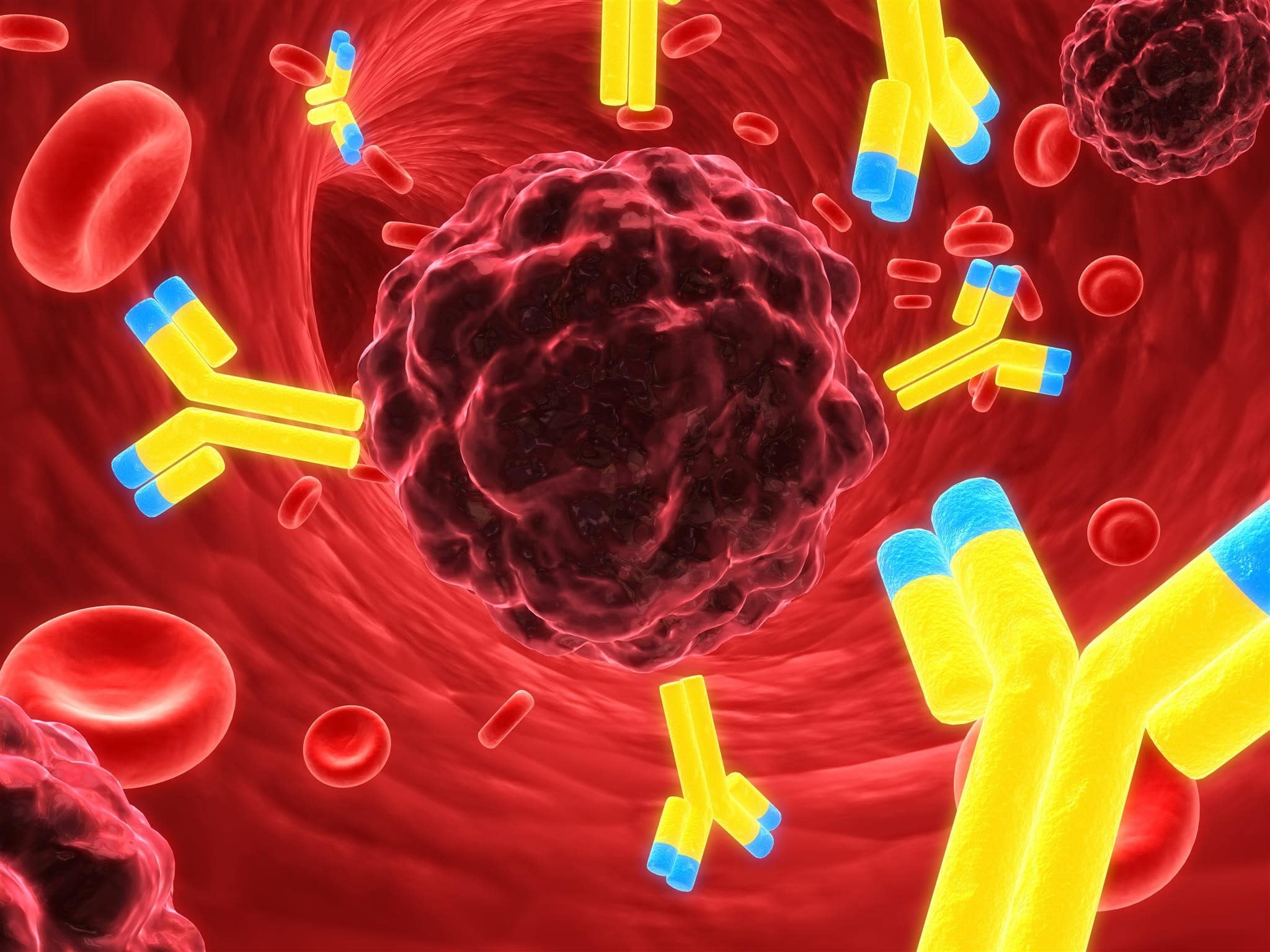
Antibody Drug Conjugates: The New Wave of Pharmaceuticals
Antibody Drug Conjugates (ADCs) are the new class of highly potent biopharmaceutical drug with high specificity for cancer therapy. The design and synthesis of an effective ADC is challenging, requiring specialized equipment for the concept to be a reality.
Blood Cell Labeling 101
Variety of methods for the labeling of erythrocytes (red blood cells or RBC), leukocytes (white blood cells or WBC), and thrombocytes (platelet) have been established, employing either Tc-99m or In-111 as two of the most common radionuclides

Esco Healthcare Welcomes 2018!
Esco Healthcare, a member of the Esco Group of companies, is comprised of 3 divisions namely, Esco Pharma, TaPestle Rx, and Esco VacciXcell. Altogether, these 3 divisions aim to make your 2018 a year of continuous learning for unsurmountable growth and development.

Ketamine: The Solution To Stopping Suicide?
Ketamine, a N-methyl-d-aspartate antagonist, is a legal prescription medication indicated as a painkiller, sedative, anesthetic, and anti-depressant. Ketamine’s use as an antidepressant and a post-traumatic stress disorder (PTSD) treatment is gaining more attention, and so further investigational researches are being conducted.
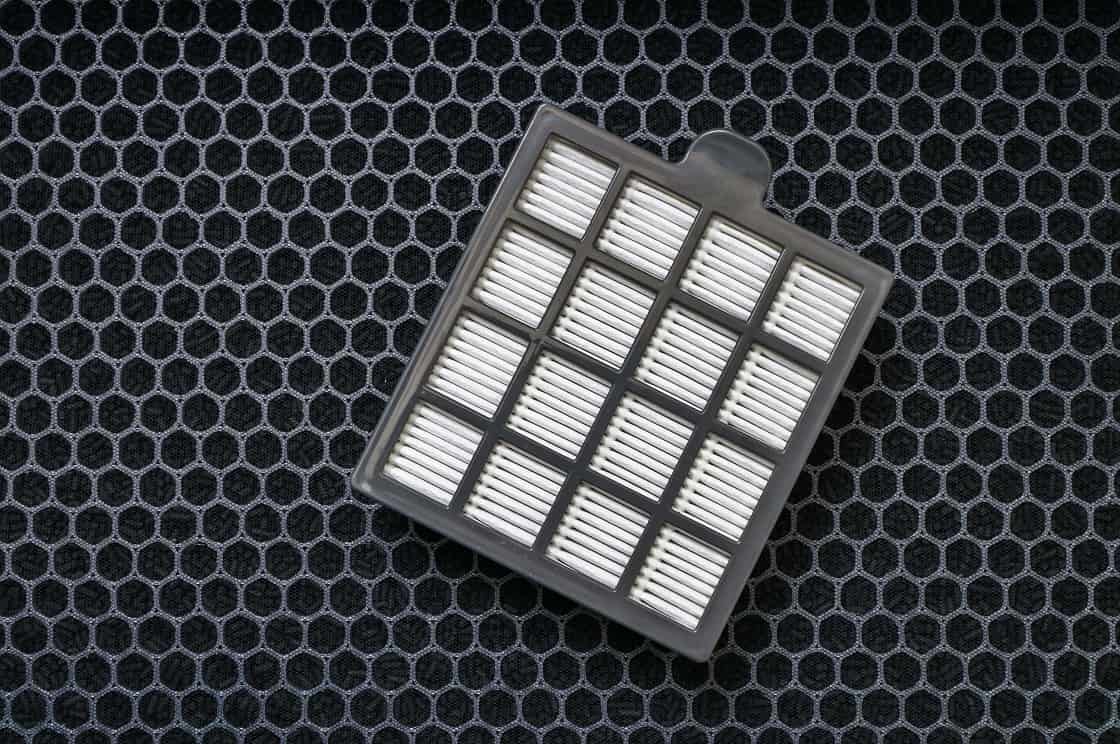
Survivor 101: Safe Change Filtration
Safe change filtration was originally designed to handle radiopharmaceuticals for the nuclear market. In more recent times, this technology has been extended and utilized for the handling of most pharmaceutical compounds.





