How Clean is Clean Enough?

(BioVap™ Bio-decontamination System)
Bio-decontamination 101
- Hydrogen peroxide (H2O2) is a highly effective sterilant being used in the disinfection of packaging materials and in processes where sterilisation is required. It has a broad antimicrobial activity spectrum and shows low toxicity.
- Residues of H2O2 decompose into neutral species, which underlines the environmental friendliness of this agent.

Source: http://brilliantbiologystudent.weebly.com/effect-of-temperature.html
- For complex geometries disinfection with liquid peroxide has limits. For this case H2O2 in the vapor state has been widely used especially within the pharmaceutical and healthcare industries in many years.
- The efficacy, cleanliness, and overall performance for the bio-decontamination system are validated using biological indicators such as Geobacillus stearothermophilus spores.
Chemistry / Biocidal Effect
- The hydrogen peroxide molecule has a unique geometry with a high-energy angle, which makes splitting of O-O-bond easy.
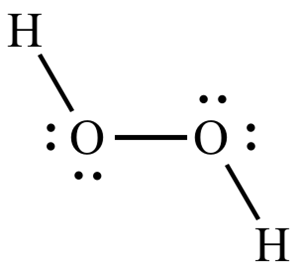
- The biocidal effect of hydrogen peroxide comes from the oxygen atom/radical, which is released under influence of the enzyme catalase at the microbial surface. The oxidative power of oxygen radicals cause irreversible damage to enzymatic systems and microbial DNA.
Regulatory

“Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice” published by FDA in September 2004, within Appendix 1: Aseptic Processing Isolators explains the need for decontaminated surfaces:
“1. Surface Exposure
Decontamination procedures should ensure full exposure of all isolator surfaces to the chemical agent. The capability of a decontaminant to penetrate obstructed or covered surfaces is limited….”
“2. Efficacy
The decontamination method should render the inner surfaces of the isolator free of viable microorganisms. Multiple available vaporized agents are suitable for achieving decontamination…. Cycles should be developed with an appropriate margin of extra kill to provide confidence in robustness of the decontamination processes. Normally, a four- to six-log reduction can be justified depending on the application.”
“E. Filling Line Sterilization
…Where decontamination methods are used to render certain product contact surfaces free of viable organisms, a minimum of a six-log reduction should be demonstrated using a suitable biological indicator.”

PIC/S Recommendation “Isolators used for Aseptic Processing and Sterility Testing”; Chapter “An expansion of the detailed points to be considered for the implementation of the principles to isolators subjected to a sporicidal process” collects 14 points for the sporicidal process. Point 9.4.1 mentions H2O2 bio-decontamination:
“…The agent selected for gas generation should be sporicidal. The agent used for gas generation or other means of application should be capable of rapidly killing bacterial endospores, fungal spores and vegetative microorganisms. … Peracetic acid, hydrogen peroxide and formaldehyde are used. …”
What is BioVap™?
Esco Pharma has developed an effective hydrogen peroxide based bio-decontamination system capable of achieving a 6-log reduction in bio-burden validated using stainless steel ribbons populated with Geobacillus stearothermophilus spores as biological indicators.
Science Behind the Process:

The Esco BioVap™ system is a process of atomizing the hydrogen peroxide sterilant creating a dry fog as it is injected into the space. This unique system (patent pending) creates a charge on the atomized droplets as it passes through the nozzle generating droplets of the sterilant to contain billions of reactive molecules to execute a microbial kill. Also, the mutual repulsion of the charged droplets contribute to the superior distribution of the sterilant. Due to the attraction to the uncharged surfaces, the droplets burst on impact and immediately start the sterilisation process.
The volume of sterilant used therefore is minimised and can be of less concentration unlike conventional vaporising systems.
This revolutionary bio-decontamination system is not affected by the temperature or relative humidity. Therefore, there is no requirement to pre-condition the space being bio-decontaminated which leads to reduced cycle.
Conventional Gaseous Systems
| Pre-conditioning | Gassing / Injection | Dwell | Aeration |
Esco BioVap™ System
| Gassing / Injection | Dwell | Aeration |
Flexibility Features
Esco BioVap™ system is developed to be flexible enough to adapt to different requirements of customers and facilities, from cabinets and transfer hatches up to modular enclosures to isolators.
BioVap™ apt for Esco Isolators!
References:
http://www.gmpua.com/Equipment/Sterility/Isolator/Bio-DecontaminationH2O2.pdf
http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070342.pdf
https://www.picscheme.org/layout/document.php?id=158
For more information, our brochure for BioVap™ Bio-decontamination System or Meet us at these events:
|
Pharmtech Expo 2016 |
November 22 - 25 |
Crocus Expo IEC, Pavilion 2, Moscow, Russia |
|
MENA Pharmaceutical Manufacturing Congress |
November 29 - December 1 |
Le Meridien Jeddah, Jeddah, KSA |
|
2016 ASHP Midyear Clinical Meeting and Exhibition |
December 4 - 8 |
Mandalay Bay Convention Centre, Las Vegas, Nevada |





