Biodecontamination Efficiency: Get to Know the BioVap™
Contamination control is of extreme importance in biopharmaceutical industries, especially in maintaining product quality and sterility. As standards and guidelines evolved over time, biodecontamination became a part of the standard operating procedures (SOPs) for facilities who manufacture and handle high-quality products.
One of the most used decontamination agents is hydrogen peroxide (H2O2) for surface decontamination of both sterile and containment processes. This is mainly due to its efficiency, material compatibility, and safety. Vaporized H2O2 is widely used in different applications in the pharmaceutical and life science industry. A wide variety of technologies have been developed for the improvement of delivery and efficacy of H2O2 in terms of superior biodecontamination.
Esco Pharma has developed an effective hydrogen peroxide-based bio-decontamination system capable of achieving a 6-log reduction in bioburden. The spore log reduction is validated by a biological indicator, Geobacillus stearothermophilus spores utilizing stainless steel ribbons.

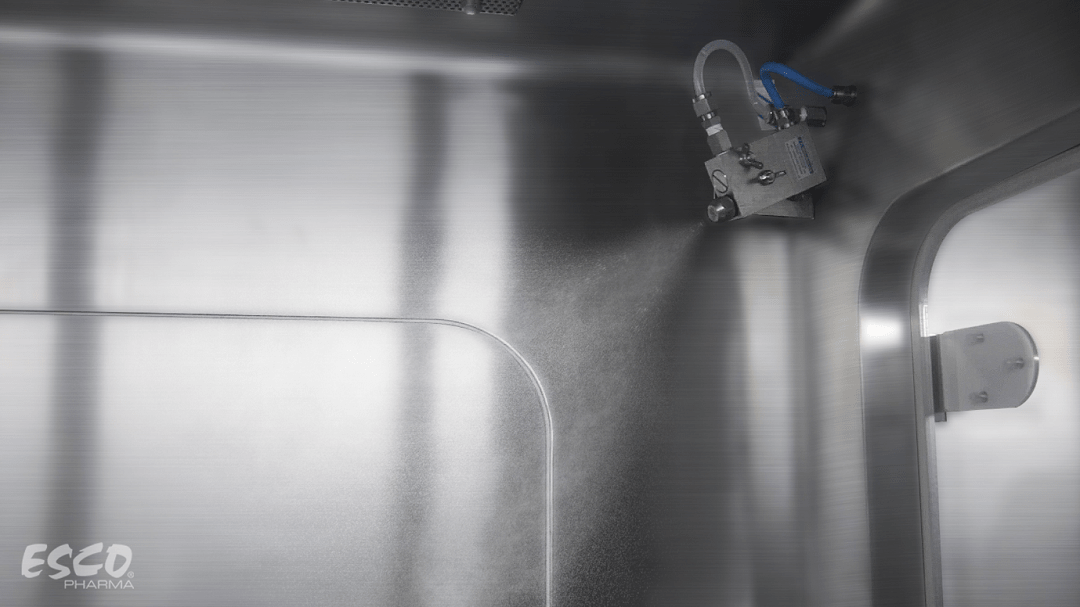
Figure 1. Esco BioVap™ Biodecontamination System
The BioVap™ has been developed in response to increasing demands from the pharmaceutical, biotechnological, and other related industries in need of more stringent requirements on decontamination. Hydrogen peroxide breaks down into oxygen and water upon the completion of the sterilization process which makes it one of the most environment-friendly decontamination agent available.
The BioVap™ is developed for performing biodecontamination of aseptic barrier system, pass through systems, biological safety cabinets, cleanrooms, and any other space where surface sterility is required.
Science Behind the Process
The BioVap™ system is a process of atomizing the H2O2 sterilant by utilizing a mist fog as it is injected into an enclosed space. This unique system creates a charge on the atomized droplets as it passes through the nozzle.
This charge imparted on the droplets of sterilant creates two important phenomena:
-
a. Each droplet of the sterilant contains billions of reactive molecules to execute the microbial kill.
-
b. Through mutual repulsion, the droplets repel each other and distribute quickly through the space achieving a superior distribution of the sterilant. The charged droplets are attracted to the uncharged surfaces within the space. Upon impact, the droplets will burst immediately, initiating the sterilization process.
For conventional vaporizing systems, the sterilant is evaporated first, making the process more time-consuming. But since BioVap™ converts the sterilant to atomized mist fogs, the evaporation stage is eliminated for a more direct process. Moreover, the system is not affected by temperature or relative humidity so there is no need to precondition the space prior to the decontamination procedure. The atomization of the sterilant and the elimination of the preconditioning stage makes BioVap™ more cost-effective and more energy efficient.
Process Step
Prior to normal run operations, the chamber must first be decontaminated to obtain a sterile working environment. The internal conditions of the process chamber must meet all the standard conditions during operations for aseptic processing. Biodecontamination is also applicable for the prevention of false negative results during sterility testing.
The BioVap™ technology has the cycle conditions set prior to sequence initiation. Multiple cycles are capable of being set and stored under the system. There are up to 10 available cycles which can be configured independently.
-
a. Priming
H2O2 will be pumped from the sterilant bottle or container to the loading station. The purpose of this stage is to fill the line with H2O2 and remove any air pockets.
-
b. Injection
H2O2 will be pumped and injected to the chamber in the form of atomized mist. The compressed air injected via a nozzle will break down the H2O2 into a very fine mist of micrometer sizes. The slightly positive charge of H2O2 will attract the negatively charged conductive surface inside the chamber following distribution.
-
c. Dwelling
The injection of H2O2 and compressed air will stop while keeping the chamber close. This is an idle step to provide sufficient exposure of H2O2 to the surface.
-
d. Aeration
The injected H2O2 will be released to the isolator’s exhaust system. The control system will activate the fan blowers to exhaust the air outside and to intake fresh air to the chambers. Vaporized H2O2 sensing will start after an adjustable time delay to ensure that the chamber has a residual H2O2 concentration of less than 1.0 ppm.
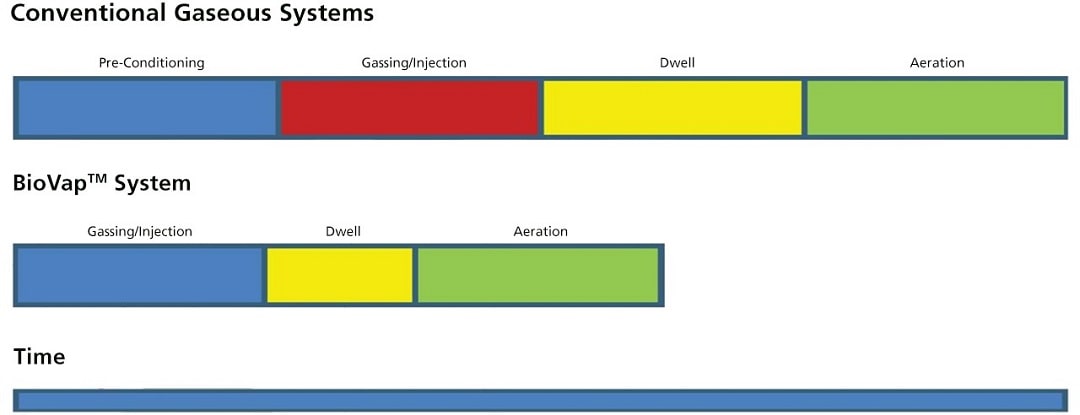
Figure 2. Conventional Gaseous Systems vs. BioVap™ System
Flexibility Features
Esco BioVap™ system is developed to be highly flexible and can be customized into various equipment such as biosafety cabinets, pass boxes, modular enclosures, and isolator systems.

Figure 3. General Processing Platform Isolator (GPPI) with integrated BioVap™- System

Figure 4. BioPass™ Pass Through with BioVap™ System
References:
1. Feinstein, S., et al. (2019) Vaporous biodecontamination: A matter of efficiency. Accessed 19 June 2019 from https://www.cleanroomtechnology.com/news/article_page/Vaporous_biodecontamination_A_matter_of_efficiency/151247
About our BRANDS
Esco Pharma provides specialist services, equipment packages, and process solutions from our core platform products leading to improved operator protection, reduction of cross contamination, and more efficient processing, thereby directly and indirectly advancing occupational health and human healthcare.
About Esco Pharma
Esco Pharma’s largest global network of localized application specialists and service offices provides faster response and local service translating into more competitive costs on maintenance, and shorter project life cycles..
Esco provides standardized platforms with inbuilt configurations without constraints on operational parameters. This enables pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with international standards for occupational health and safety.





