Large Molecule | Esco Healthcare
A Curtain Raiser for Large Molecule!
Biologics are medications targeting specific genotypes or protein receptors. In contrast to most chemically synthesized drugs with clearly defined structure, most biologics, due to their large and complex molecules, are not easily identified or characterized. It is also because of their size that biologics are usually administered via injection or infusion rather than oral administration. They are obtained through isolation from various natural sources (e.g., human, animal, or microorganism), and can also be produced by biotechnological methods and other cutting-edge technologies.
Biological products are diverse and wide-ranging, which may include vaccines, blood and blood components, allergenics, somatic cells, gene therapy, tissues, and recombinant therapeutic proteins. According to the Food and Drug Administration (FDA), although complex and tedious to work on, biologics in time, may offer the most effective means to treat various medical illnesses and conditions for which no other treatments are available.
Additionally, since these products tend to be heat sensitive, and susceptible to microbial contamination, aseptic technique must be observed in all stages of drug manufacture. Biologics are also being regulated by the Food and Drug Administration (FDA), specifically by the Center for Biologics Evaluation and Research (CBER). CBER is one of the centers within FDA, and is an agency within the United States Government’s Department of Health and Human Services (HHS), that aims to protect and advance the public health by ensuring that the biological products are safe, effective, and available to those who are in need.
A Quick Run-Through of How Large Molecules are Made
In understanding large molecule production, it is important to first comprehend its meaning. Large molecules, also known as biopharmaceuticals, biologicals, or biologics, point to medicinal products with therapeutic effects that can be composed of proteins, nucleic acids, sugars, living cells, or even tissues. Produced using various sources and methods—such as non-engineered biological sources, blood, and its derivatives, or by genetic engineering in recombinant technology—biologics cover a wide variety of products, including vaccines, therapeutic antibodies, allergens, and gene therapy.
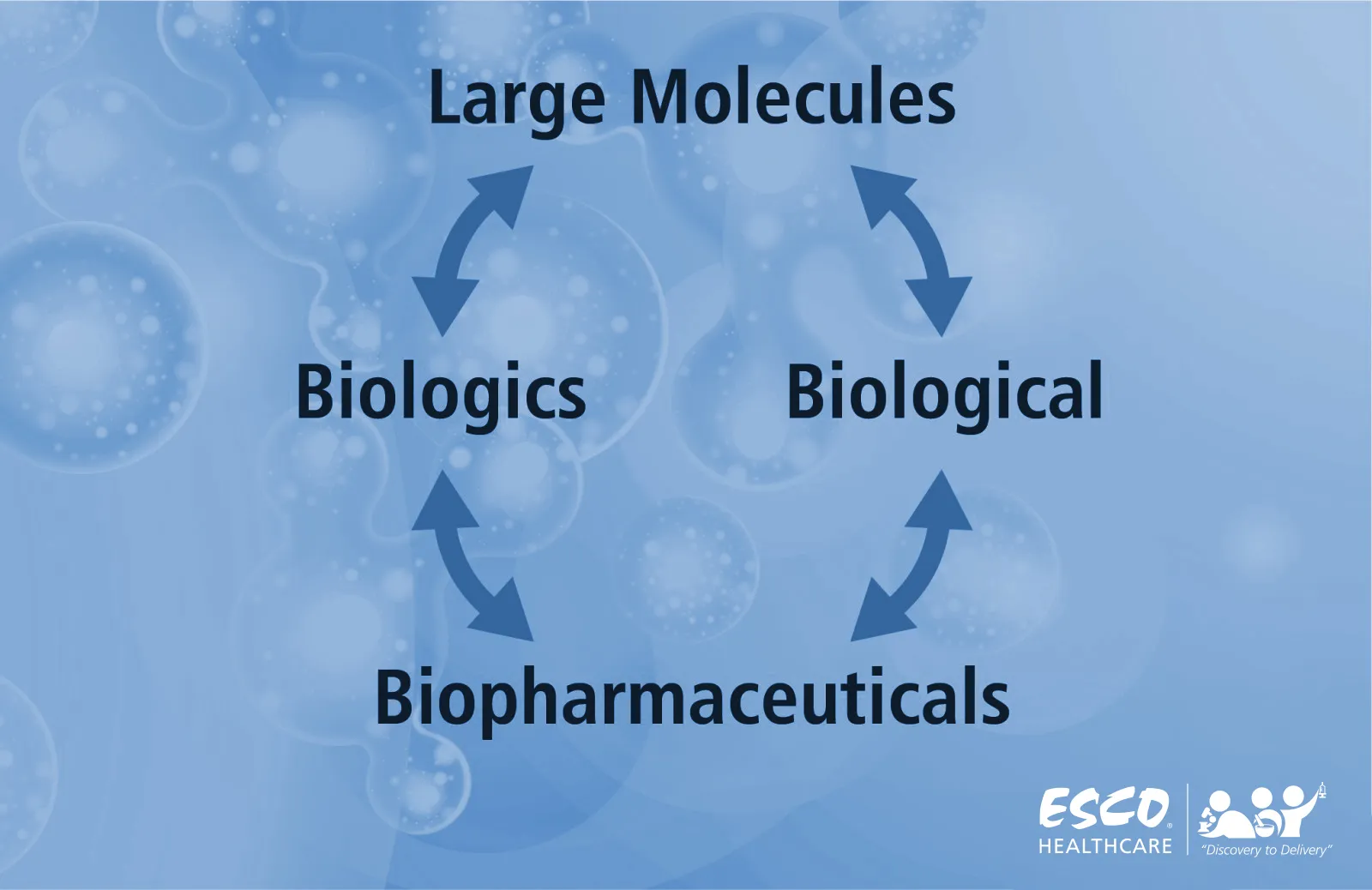
Fig 1. Interchangeable terms of Large Molecules, Biologics, Biological, and Biopharmaceutical
Interest in vaccines has skyrocketed as a result of the increased public awareness due to the COVID-19 pandemic, thus more research is being performed in this area of study. The viral vector vaccine is one vaccine technology that is currently evolving quickly. This is because the viral vector, which can be modified to produce therapeutic genes, is the most efficient method of gene transfer for altering certain cell types or tissues. In order to transmit genes to cells for either temporary or permanent transgenic expression, several viral types are presently being researched. Inserting and delivering genetic material into cells in cell culture is another application for viral vectors.
Another source for large molecule drugs is a wide range of recombinant expression systems. Most recombinant proteins that have received marketing authorization to date are generated using recombinant Escherichia coli or recombinant mammalian cell lines, such as Chinese hamster ovary (CHO) or baby hamster kidney (BHK). Yeast cells, in particular Saccharomyces cerevisiae, which exhibits a variety of qualities that make them appealing in this context, are another existing production system.
As for the production process of the final large molecule product, it can further be defined as upstream and downstream processing, where upstream processing refers to the initial fermentation procedure that yields the first product generation. On the other hand, downstream processing is the actual purification of the protein product and the development of the completed product format, which includes filling it into its final product containers, freeze-drying it (if a dried product format is needed), and finally sealing the containers.
During the production phase, while the majority of biopharmaceuticals or large molecules manufacturing process takes place in specialized clean areas (much like most pivotal manufacturing processes for parenteral pharmaceuticals), proper design, use of primary engineering control, and maintenance are also critical to ensure the overall safety of the final product. A clean air environment with a Grade A or ISO Class 5 classification is required for all open handling of large molecule drugs in order to safeguard the product from contamination as it is meant to be sterile.
Even so, maintaining the sterility of parenteral large molecules is a challenge. There’s a great chance of product contamination when large molecules are sterilized by filtration and aseptically filled into sterile final product containers afterwards. Thus, as is the case with small molecule drugs, the finished-product sterility test marks one of the most important product tests carried out by QC.
Large Molecule vs Small Molecule Drugs
The breakthrough of organic synthesis revolutionized the pharmaceutical sector by enabling companies to manufacture any desired molecule for use in almost all therapeutic fields. These small molecules, which vary in complexity but generally weigh less than 900 Dalton (Da), have been the focus of therapeutic development for decades. For instance, the most widely used COX-2 inhibitor, Paracetamol, weighs only 151 Da.
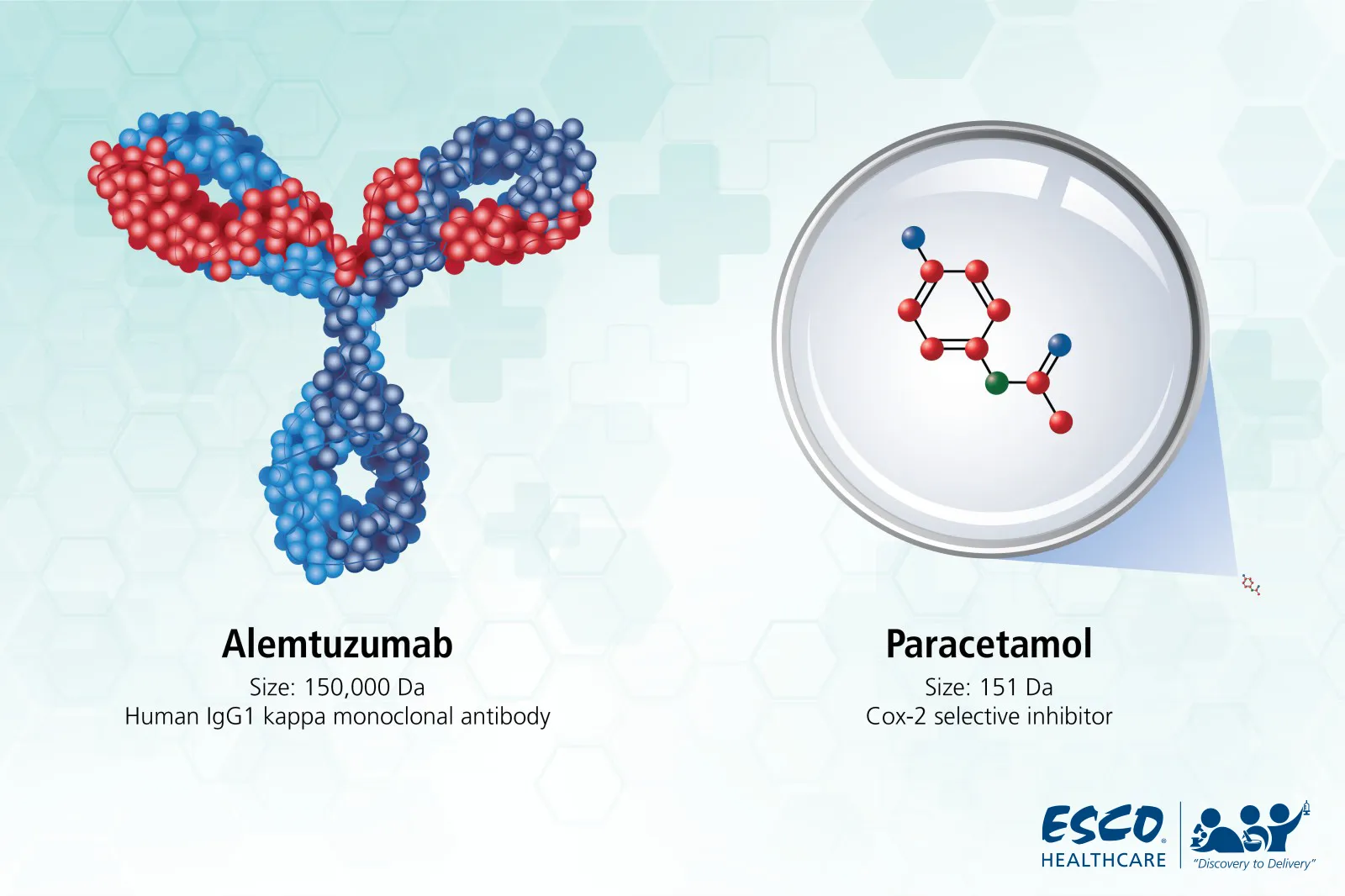
Fig 2. Molecular size comparison of Paracetamol (small molecule) and Alemtuzumab (large molecule)
The majority of small molecule medications are synthesized chemically, although small molecules with more complicated structures, such as paclitaxel with 854 Da molecular weight, are generated recombinantly in cell cultures. Most of these medications are created and packed into orally administered pills, through which they are delivered throughout the body. Small molecules often exert their effects by penetrating cells, causing the desired biological response. Overall, the pharmaceutical business relies heavily on this traditional method of drug discovery, which keeps expanding yearly.
Other parameters differentiating large and small molecules are tabulated below:
| Parameter | Large Molecule | Small Molecule |
|---|---|---|
| Molecular | Significantly larger ~ 3,000-150,000 Da | Smaller, < 900 Da |
| Source | Mostly with recombinant biotechnology | Mostly with chemical synthesis |
| Mechanism of action | Mostly act through external cellular binding to induce the desired cellular response | Mostly act through cell penetration, prompting the desired cellular responses |
| Administration and formulation | Mostly parenteral/injection, lower flexibility in formulation | A variety of oral, topical and parenteral, flexible in the formulation |
| Cost | Significantly higher in development, manufacturing and final product | Relatively lower |
| Market Domination | Less than 10% | Around 90%* |
| Structure | Complex with several structural variations | Simple and well-defined |
| Characterization | Relatively difficult to define | Relatively easier |
| Identical to reference product | No, only biosimilar | Yes |
| Adverse immune response | Higher potential | Lower potential |
| Target-specific response | Very target-specific | More prone to induce harmful non-target effects |
| Manufacturability/ Ease of manufacture | Difficult to scale up and maintain batch-to-batch equivalence | Easier to scale up, good uniformity feasible |
Challenges in Manufacturing Large Molecule Drugs
As mentioned above, a challenge in large molecules/biopharmaceutical manufacturing is regarding the possible impurity of large molecules, resulting in contamination by microorganisms, viral particles, pyrogenic substances, DNA, and contaminating proteins, which in turn might jeopardize the product quality and induce potential adverse effect that pose a threat to patients’ safety.
Curated by Hong et al., 2018, further challenges are regarding the development of the continuous manufacturing system in biopharmaceuticals such as:
- Microscale technologies for high-speed continuous processing
- Plug-and-play modular unit operations with integrated monitoring and control systems
- Dynamic modeling of unit operations and entire biopharmaceutical manufacturing plants to support process development and plant-wide control, and
- Model-based control technologies for optimizing startup, changeover, and shutdown.
The continuous manufacturing system is a potential solution for possible impurity. Modular unit operation supports the continuous processing of large molecules that opens the possibility for future expansion and various equipment integration. This can allow samples, during both upstream and downstream processing, to stay in convenient conditions, minimizing sample contamination. The integration of equipment such as incubators, microscopes, centrifuges or even bioreactors inside a strongly and expertly engineered primary engineering control might result in a high product yield and protect the large molecules from biological contamination and possible degradation.
Esco Healthcare provides specialist services, equipment packages, and process solutions from our core platform products. Together with its wide range of innovative and turnkey solutions, backed with four (4) core technologies, Esco Healthcare enables various industries such as pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
References:
- Morrow, T. & Felcone, L. (2004). Defining the difference: What Makes Biologics Unique. Biotechnology Healthcare, 1(4), 24-26, 28-29. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564302/
- U.S. Food and Drug Administration. (2019). Center for Biologics Evaluation and Research (CBER). Retrieved from: https://www.fda.gov/about-fda/fda-organization/center-biologics-evaluation-and-research-cber
- U.S. Food and Drug Administration. (2018). What are "Biologics" Questions and Answers. Retrieved from: https://bit.ly/FDAWhatAreBiologics
- U.S. Food and Drug Administration. (n.d.). Biological Product Definitions. Retrieved from: https://www.fda.gov/files/drugs/published/Biological-Product-Definitions.pdf
- U.S. Food and Drug Administration. (n.d.). CDER World: Biologics Review. Retrieved from: https://www.accessdata.fda.gov/scripts/cderworld/index.cfm?action=newdrugs:main&unit=3&lesson=1&topic=1
- Kesik-Brodacka, M. (2018). Progress in biopharmaceutical development. Biotechnology and Applied Biochemistry, 65: 306-322. https://doi.org/10.1002/bab.1617
- Warnock JN, Daigre C, Al-Rubeai M. (2011). Introduction to viral vectors. Methods Mol Biol. 737:1-25. doi: 10.1007/978-1-61779-095-9_1. PMID: 21590391.
- Introductory Chapter: Biopharmaceuticals by Yuan-Chuan Chen and Ming-Kung Yeh (2018)
- Moo Sun Hong, Kristen A. Severson, Mo Jiang, Amos E. Lu, J. Christopher Love, Richard D. Braatz. (2018). Challenges and opportunities in biopharmaceutical manufacturing control. Computers & Chemical Engineering. Volume 110. Pages 106-114. ISSN 0098-1354, https://doi.org/10.1016/j.compchemeng.2017.12.007
- Walsh. G. (2003), Biopharmaceuticals: Biochemistry and Biotechnology, 2nd Edition.
Recommended Products