The manufacturing process of preparing radiopharmaceuticals should meet Good Manufacturing Practice (GMP) criteria. The institution must ensure that these substances are of high-quality and will not be contaminated with any long-lived impurities after the period of their decay.
It must be noted that the radionuclide composition of some preparations is determined via the chemical and isotopic composition of the target materials. Trial preparations are advisable especially when new batches of target material are used.
The general way to manufacture radionuclides to be used for radiopharmaceutical preparations are as follows:
- Nuclear fission: During this process, an atom’s nucleus breaks up into two lighter nuclei. This process is usually observed in nuclides with high atomic numbers: i.e., the fission of Uranium-235 using neutrons in a nuclear reactor.
Nuclear fission can either happen spontaneously or with the excitation of the nucleus. The latter method can be achieved by using neutrons, protons, deuterons, or alpha particles; it can even be done via electromagnetic radiation using gamma rays.
A large amount of energy is released during the fission process, causing the production of radioactive substances and the emission of several neutrons. Said neutrons can further induce nuclear fission on nearby fissionable nucleuses, thereby, causing a chain reaction. This chain reaction is then responsible for the release of enormous amounts of energy, which if left uncontrolled, can lead to the production of an atomic bomb. In nuclear plants however, such chain reaction is controlled for societal benefits such as in the production of electrical power.
In the laboratory setting, nuclear fission can result to radioisotopes that can be used for diagnostic and/or treatment procedures. Such a controlled process must be done inside an equipment designed to handle radioactive substances, and all personnel involved must have had proper training. - Charged particle bombardment: In this process, radionuclides are produced by bombarding target materials with charged particles inside particle accelerators like the cyclotron.
- Neutron bombardment: Similar to the above method, radionuclides can be produced by bombarding the target material with neutrons, and this is usually seen in nuclear reactors. Basically, the energy of the incident particle together with the isotopic composition and purity of the target material plays a significant role in triggering the desired nuclear reaction.
- Radionuclide generator system: Radionuclides with short half-lives can be produced using a radionuclide generator system. Radionuclide generators, also referred to as ‘cows,’ contain a long-lived radionuclide – or ‘parent nuclide’- which will then decay into a short-lived radionuclide of interest known as ‘daughter nuclide’. These daughter nuclides obtained from such generators have a significant use in the medical diagnosis (imaging scans), treatment, or any other radionuclide tracer applications.
Two types of parent-daughter generator systems:
- Transient Equilibrium Generator – This generator contains a parent radionuclide with a half-life greater than that of the daughter’s. This is generally seen in generators for 99Mo/99mTc which relies on the availability of the long-lived parent radionuclide decaying further into a daughter substance with shorter half-life but with beneficial physical and decay properties.
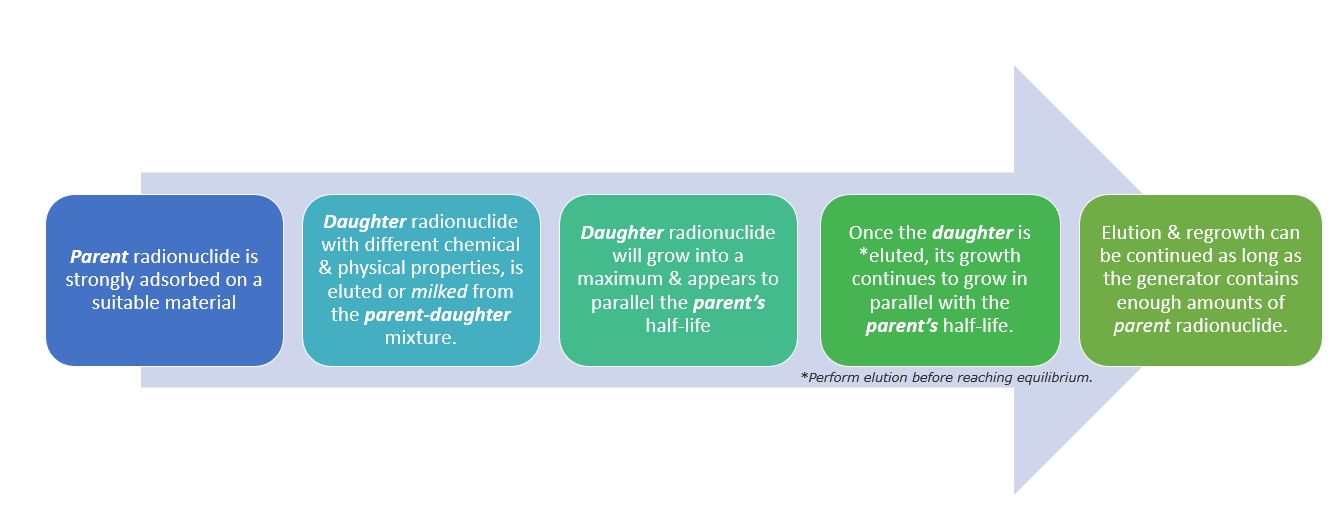
Figure 1. Events inside a Transient Equilibrium Generator.
- Secular Equilibrium Generator – The concept used here is that even the short-life of the parent radionuclide is longer than the daughter’s. There will not be a noticeable decay in the parent’s half-life even with the production of many daughter radionuclide.
The rate of daughter radionuclide production is initially greater than its rate of decay, the daughter will further increase until it reaches an equilibrium wherein its rate of production is equivalent to its rate of decay (daughter decays with a half-life similar to the parent’s).
Guidelines recommend that all materials and excipients (starting materials including precursors for syntheses) for the production of radiopharmaceuticals, are of high quality. Since the amount of the radioactive material is relatively small as compared to its excipients, the latter can have a significant influence on the overall quality of the final preparation. The excipients contained in a radiopharmaceutical preparation must be permitted by general monographs, and they must be used according to the required dosage form.
- Transient Equilibrium Generator – This generator contains a parent radionuclide with a half-life greater than that of the daughter’s. This is generally seen in generators for 99Mo/99mTc which relies on the availability of the long-lived parent radionuclide decaying further into a daughter substance with shorter half-life but with beneficial physical and decay properties.
- Sterilization: Sterile radiopharmaceuticals for parenteral route should be properly sterilized, and whenever possible, terminal sterilization is recommended. For most preparations though, filtration is the method of choice.
Notably, all sterilization methods must be validated prior to use. If there is a small batch size of preparation (one or few samples with very short half-lives), the entire manufacturing process must be validated. - Antimicrobial preservatives: Most radiopharmaceutical injections are released in multidose containers, and as a general practice, these such substances should normally contain an antimicrobial preservative. But this is not always the case for most radiopharmaceuticals; whether or not a preparation contains an antimicrobial preservative, it should be stated on the label accordingly.
Radiopharmaceutical injections with shelf-life >1 day inside multidose containers should have a suitable antimicrobial preservative.
References:
- https://www.nibib.nih.gov/science-education/science-topics/nuclear-medicine
- https://www.who.int/medicines/publications/pharmacopoeia/Radgenmono.pdf
- https://humanhealth.iaea.org/HHW/Radiopharmacy/VirRad/Eluting_the_Generator/Generator_Module/Principles_of_radionuclide_generators/index.html
Recommended Products










