Biorisk management is a full spectrum of safety and security measures carried out to effectively manage the risks accompanied by working with infectious agents and toxins in laboratories. This ranges from standard operating procedures (SOPs) to physical measures to individual practices in the laboratory.
Biocontainment, a component of biorisk management, focuses on addressing the design of safety equipment and laboratories to effectively confine infectious agents and toxins, and prevent their accidental release. Biocontainment is used to facilitate in the reduction of potential for exposure of laboratory workers or persons outside the laboratory, and the likelihood of accidental release to the environment.
Physical containment can be achieved through use/application of the following:
- laboratory practices
- containment equipment
- personal protective equipment (PPE); and,
- laboratory and facility design
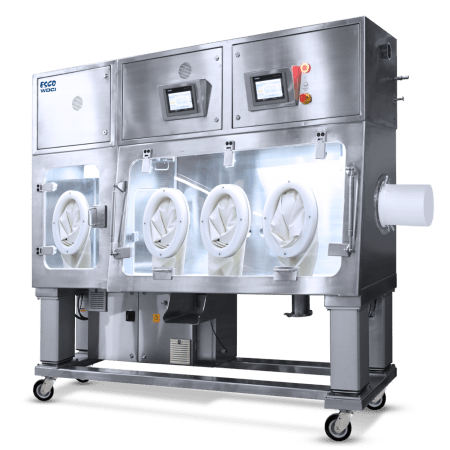
Esco Healthcare, backed with its 4 core technologies provides specialist services, equipment packages, and process solutions from its core platform products which leads to improved protection of operator, reduction of cross-contamination, and a more efficient and reliable processing.
References:
- Science Safety Security. (2019). Biocontainment. Public Health Emergency. Retrieved from: https://www.phe.gov/s3/BioriskManagement/biocontainment/Pages/default.aspx
Recommended Products