Isoclean® Healthcare Platform Isolator - Inflatable Seal – BioVap™ (HPI-IS-BVP)
Optimized Solution for Sterile/Aseptic Applications
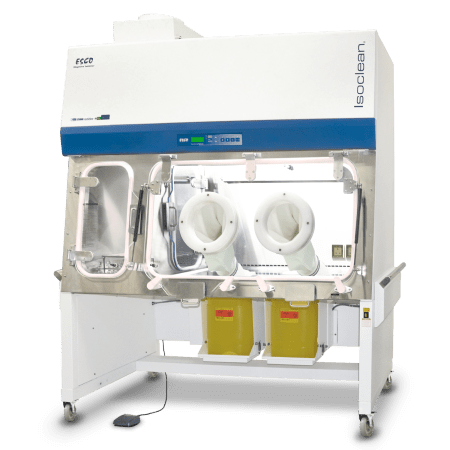
The Isoclean® Healthcare Platform Isolator – Inflatable Seal - BioVap™ Model (HPI-IS-BVP) ensures the isolation of a product/process while providing the required ISO Class 5 / Grade A environment.
HPI-IS-BVP is equipped with inflatable seals and automated dampers. As standard, the standard unit is fully integrated with both the auto-pressure hold testing and BioVap™ (hydrogen peroxide biodecontamination system with H2O2 sensors and catalytic converter).
Integration of Esco BioVap™ allows both master and independent biodecontamination process of main chamber and pass-through chambers (PTCs). This improved design facilitates ease of isolation control especially during pressure decay testing and bio-decontamination process.
This model can be adjusted on-site to operate in positive or negative pressure regime. It is also available in recirculating or total exhaust configuration.
Applications:
- Aseptic and/or Potent Compounding
- Pharmacy Compounding
- Sterility Testing
- Cell and Gene Therapy
- Peptide Production
- Biosafety Facility Level 3 or 4
- Benchtop/Small-scale Aseptic Formulation and Filling
- Small-scale Potent Material Handling
- Cosmeceutical
- R&D and Clinical Trials
- This model is capable of expanding up to 3 modules of 2-glove main chamber with 2 modules of pass-through chamber (left and right).
- Capable of automated pressure hold testing (APHT) and biodecontamination with log 6 reduction in bioburden.
- HEPA (H14) filter (as per EN 1822) with a typical efficiency of > 99.999% at 0.1 to 0.3 microns; provide superior ISO Class 5 air cleanliness as per ISO 14644-1.
- Containment enclosure classification: Class 2 as per ISO 10648-2.
- Electromagnetic interlocking doors with time delay effect ensures safety and containment during material transfer.
* With built-in air compressor to support inflatable seals in the window and dampers, and the BioVap™ biodecontamination system.
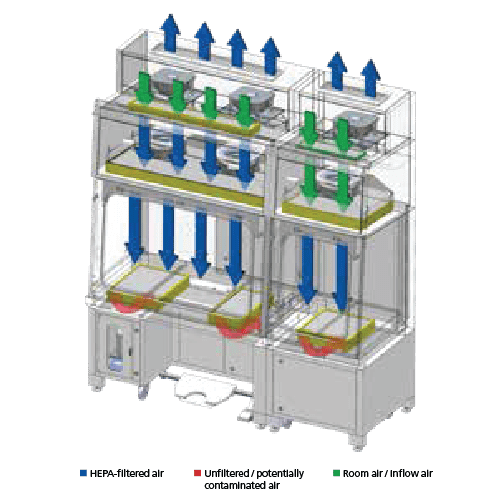
Single Pass/Total Exhaust Configuration
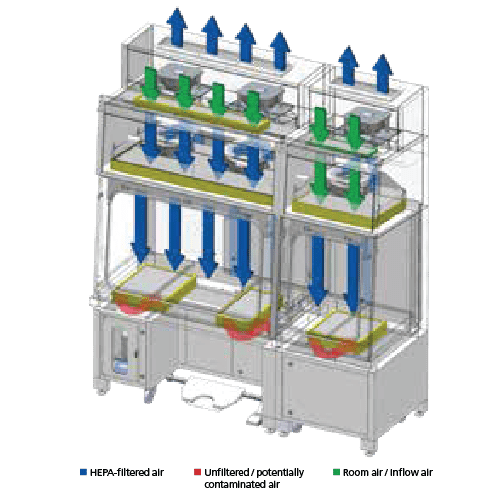
Recirculating Configuratiom
|
ISOCLEAN® |
2-glove Main Chamber |
3-glove Main Chamber |
4-glove MainChamber |
Passthrough Chamber |
3-way Passthrough Chamber |
|
|
External Dimension (W x D x H) |
1340 x 800 x 2350 mm |
1645 x 800 x 2350 mm |
1950 x 800 x 2350 mm |
730 x 800 x 2350 mm |
730 x 800 x 2350 mm |
|
|
Internal Dimension (W x D x H) |
1290 x 620 x 700 mm |
1595 x 622 x 700 mm |
1900 x 622 x 700 mm |
680 x 620 x 700 mm |
680 x 620 x 700 mm |
|
|
Passthrough Chamber - Tray Dimension |
N/A |
N/A |
N/A |
270 x 660 mm |
270 x 660 mm |
|
|
Isolator Construction |
External Body |
ISOCIDE™ Powder-coated electrogalvanized steel |
||||
|
Internal Chamber |
Stainless steel 316L |
|||||
|
Airflow |
Unidirectional/Laminar Airflow |
|||||
|
Process Chamber Pressure |
+37 Pa |
+25 Pa |
||||
|
Selectable between -60 Pa to +75 Pa |
||||||
|
Process Chamber Downflow Velocity |
0.45 m/s +/-20% |
|||||
|
Chamber Lighting |
Normal Operating Mode |
Warm White, Minimum 500 Lux |
no lighting for PTC Module |
|||
|
Bio-decontamination Mode |
Blue |
no lighting for PTC Module |
||||
|
Alarm Mode |
Red |
no lighting for PTC Module |
||||
|
Aeration Mode |
With Integrated Catalytic Converter |
|||||
|
Pressure Hold Test |
During FAT and IQOQ |
Class 2 Containment as per ISO 10648-2 |
||||
|
Automated Daily Routine |
Class 3 Containment as per ISO 10648-2 (Pressure hold test prior to each biodecontamination |
|||||
|
Net Weight |
525 Kg |
650 Kg |
|
328 Kg |
|
|
|
Inflatable Sealed Side Adaptor Plate/ Retrofit Option |
RTP for sizes 105 mm, 190 mm, or 270 mm |
N/A |
||||
|
Up to 4 x 1” Tri-clover connection |
N/A |
|||||
- Available in Recirculating or Total Exhaust configuration
- Integration of a side-mounted CO2 Incubator
- Glove Leak Tester
-
Glove Port sizes
- 300 x 300 mm
- 300 x 200 mm
- CCTV integration
- Access to rear view monitor system
- Addition of sterility test pump
- Mechanical integration of viable/non-viable particle monitoring (with separate software)